Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations

A detailed analysis of expedited regulatory review time of marketing authorization applications for new anticancer drugs in the US and EU - da Costa Gonçalves - 2022 - Clinical and Translational Science - Wiley Online Library
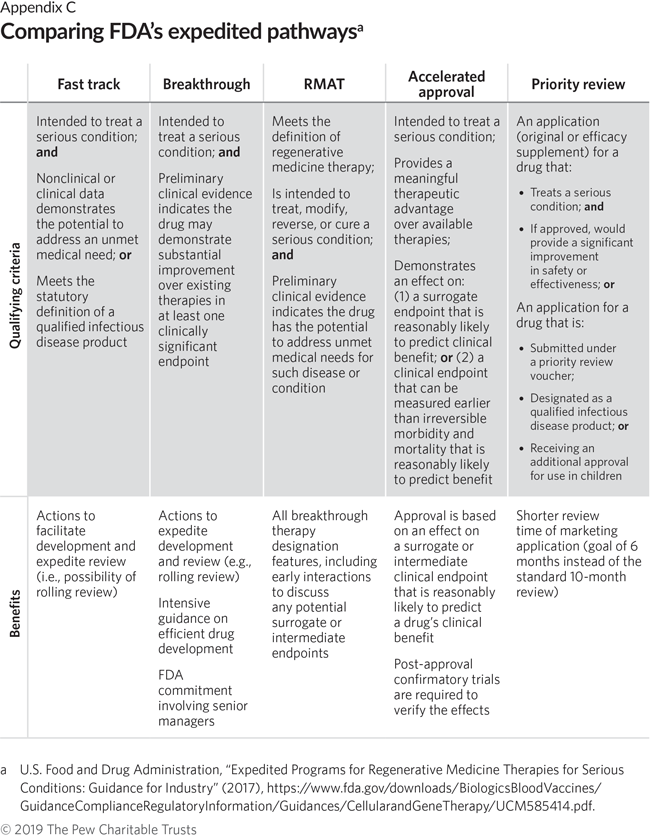
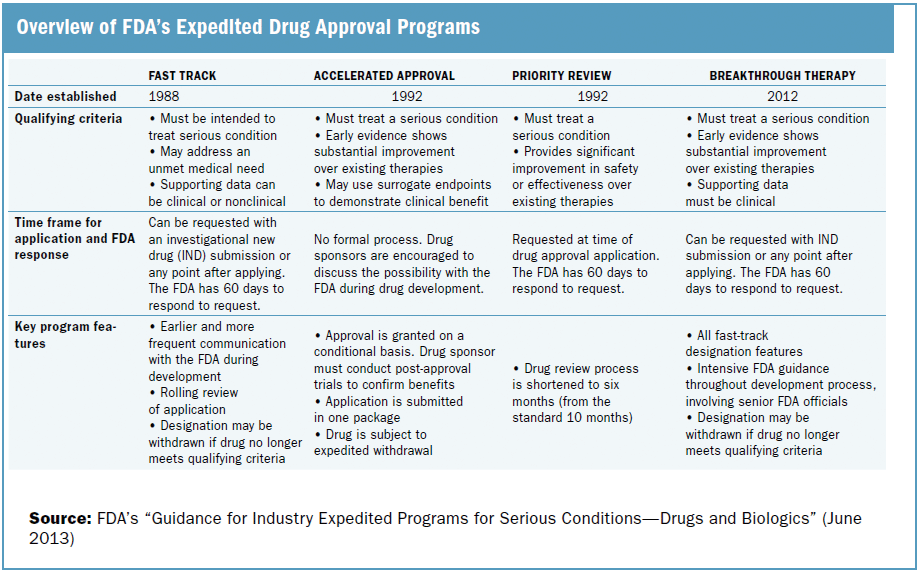
FDA's Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts

CorMedix eyes US FDA priority review for bloodstream infection drug Defencath | S&P Global Market Intelligence
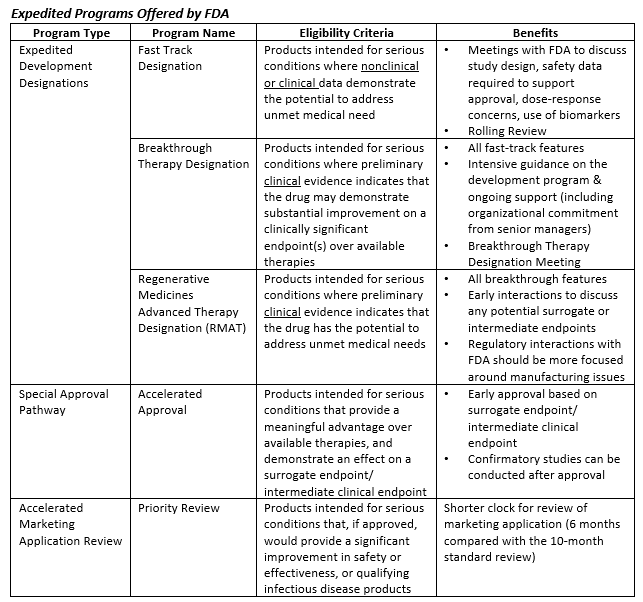
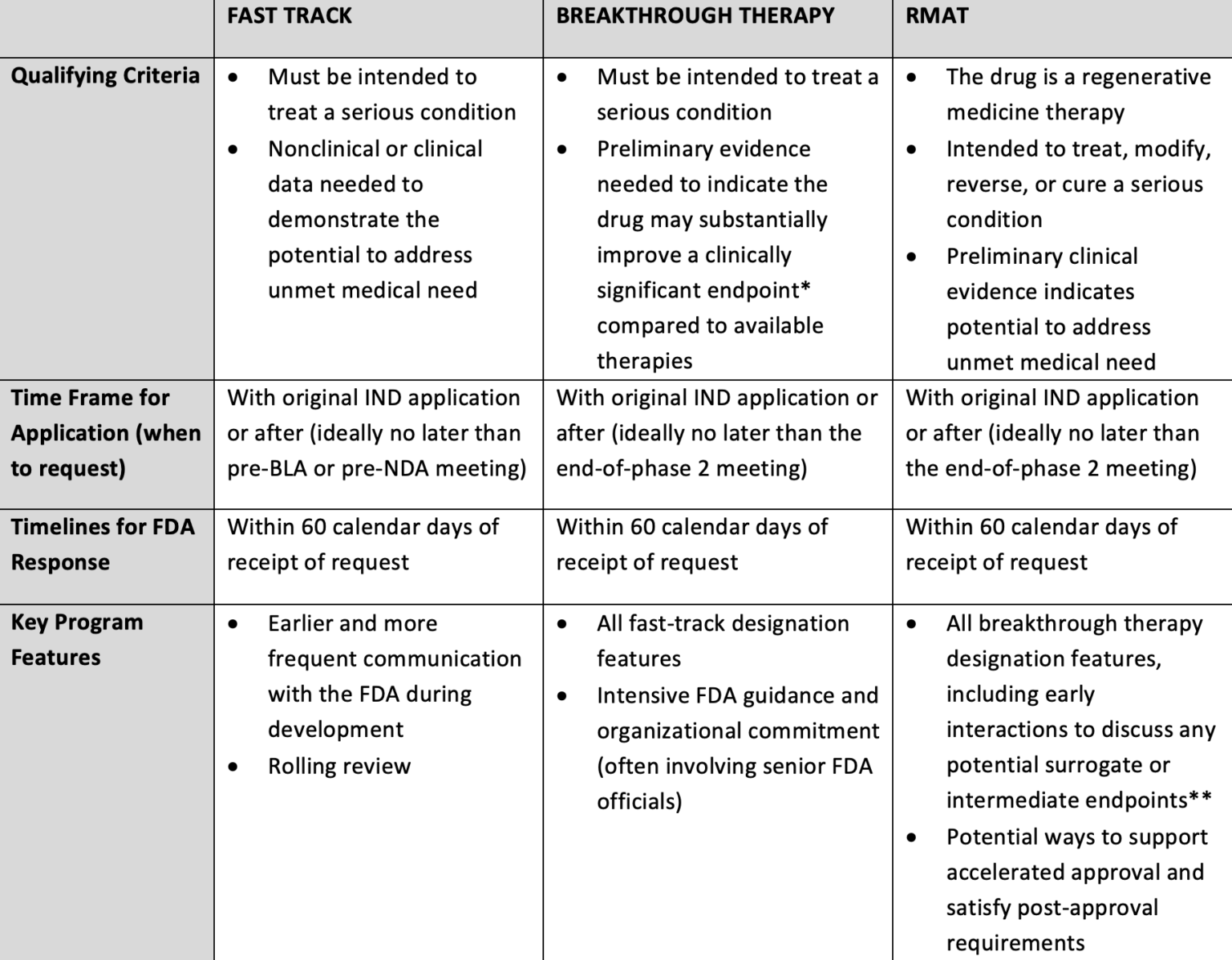
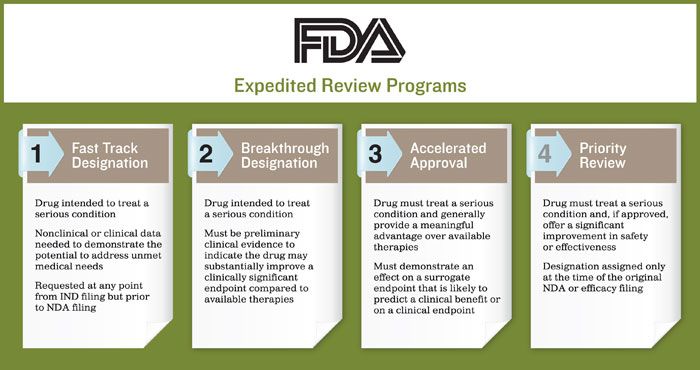
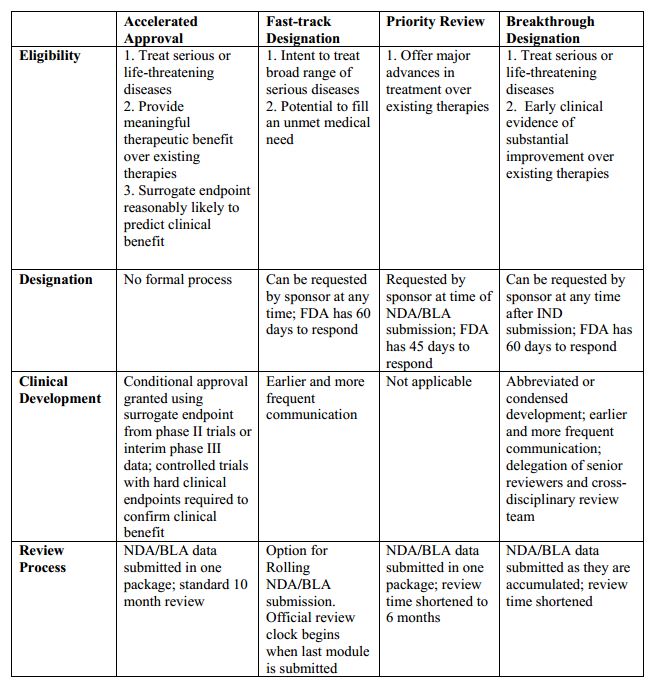
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group

Vertex/CRISPR to begin rolling review in US for gene-edited therapy; get FDA designations | Seeking Alpha
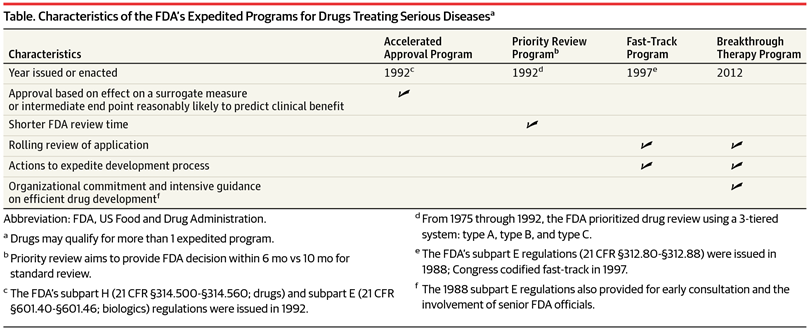
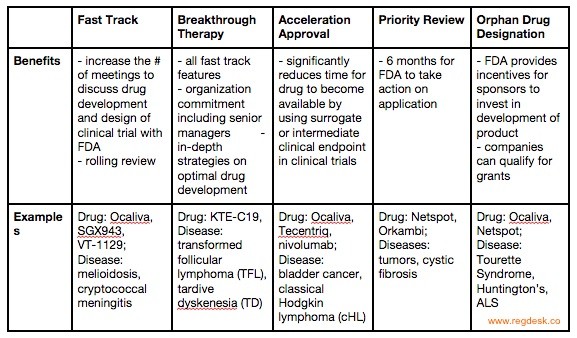
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB) | Seeking Alpha

CytoDyn Inc. submits the first of three main sections of its HIV Biologics License Application to FDA under rolling review