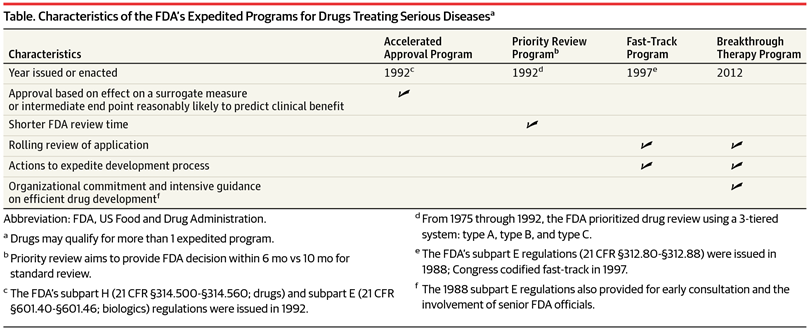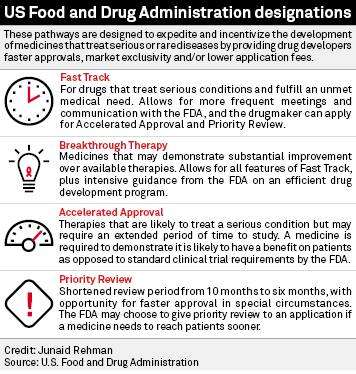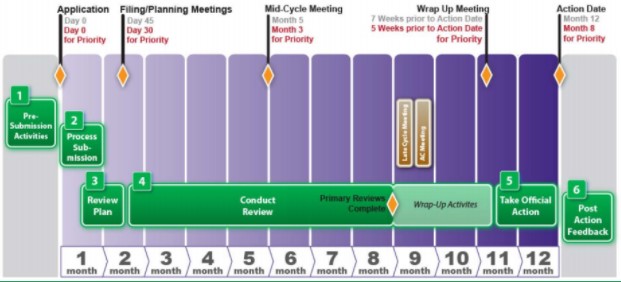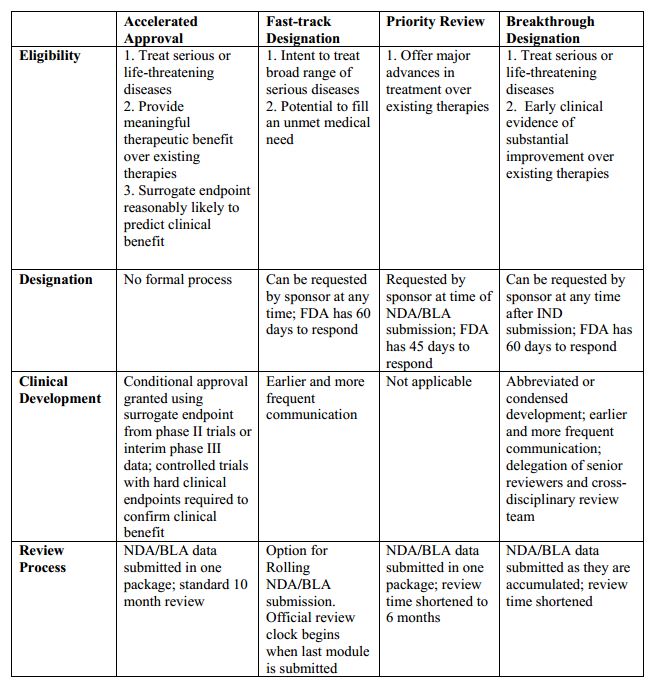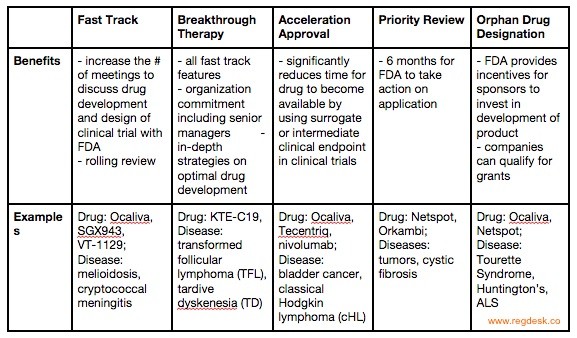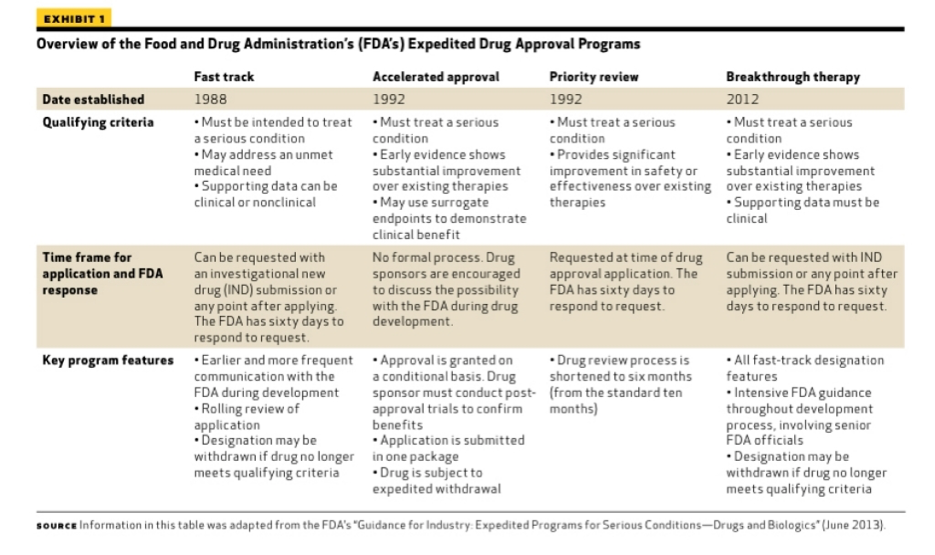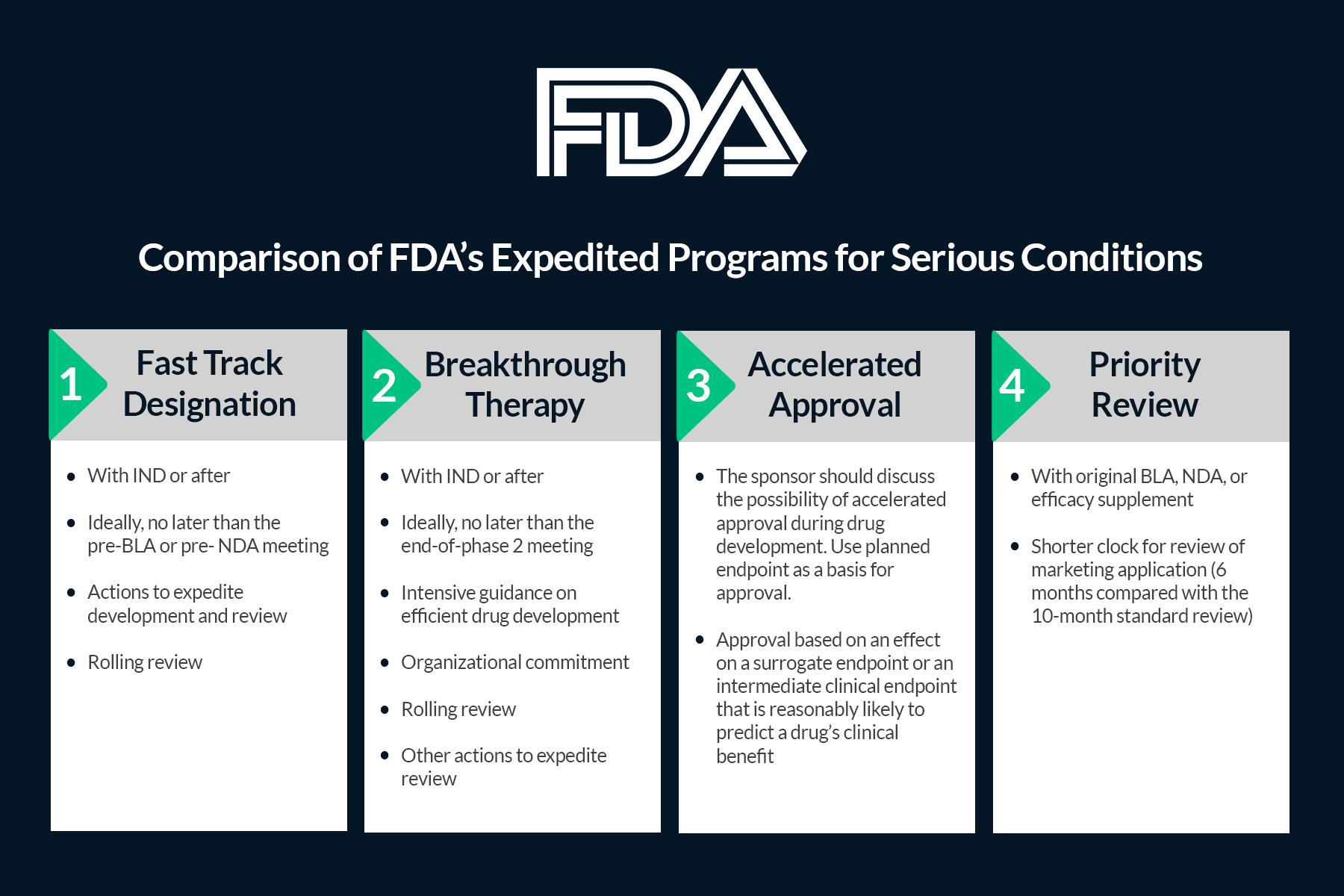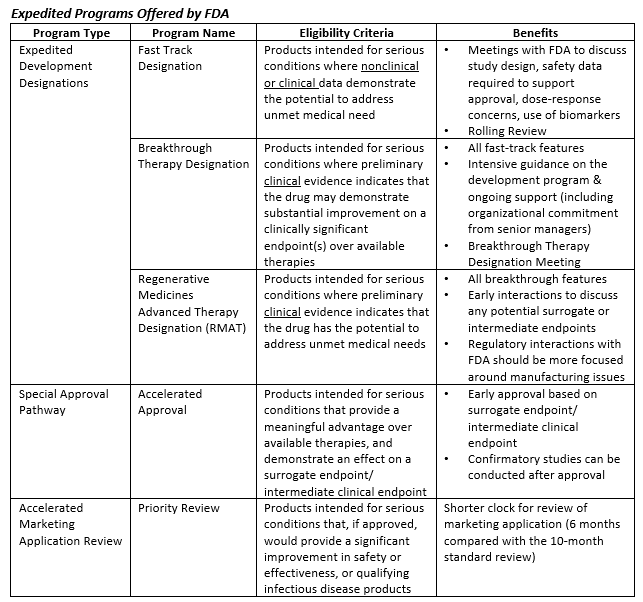
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group

CytoDyn Inc. submits the first of three main sections of its HIV Biologics License Application to FDA under rolling review
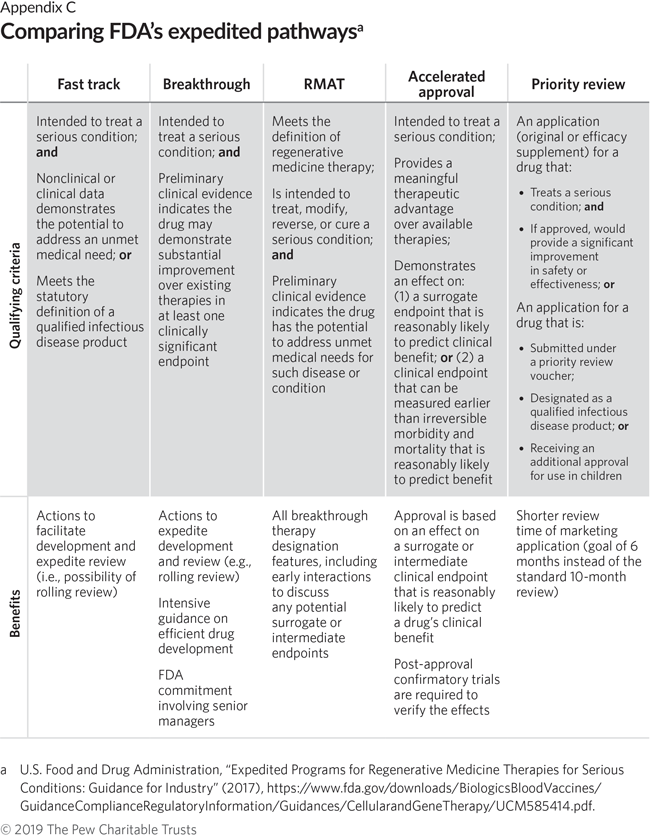
FDA's Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts

Y-mAbs Reports Initiation of Rolling Review of BLA for Naxitamab to the US FDA to Treat Neuroblastoma

Analysis of the Real-Time Oncology Review (RTOR) Pilot Program for Approvals of New Molecular Entities | SpringerLink

Update to Drugs, Devices, and the FDA: How Recent Legislative Changes Have Impacted Approval of New Therapies - ScienceDirect
FDA Moves Against Fast Track Vaccines - News about Energy Storage, Batteries, Climate Change and the Environment

A detailed analysis of expedited regulatory review time of marketing authorization applications for new anticancer drugs in the US and EU - da Costa Gonçalves - 2022 - Clinical and Translational Science - Wiley Online Library